T 1252/20 - Board of Appeal Revises Interpretation of 'Substance or Composition' for Medical Use Claims
Rechtsprechung | 22.05.2024
London partner Adam Lacy, and Italian and European Patent Attorney Arianna Bartolini review a recent decision of the EPO Board of Appeal making it easier for patentees to obtain protection for medical use claims.
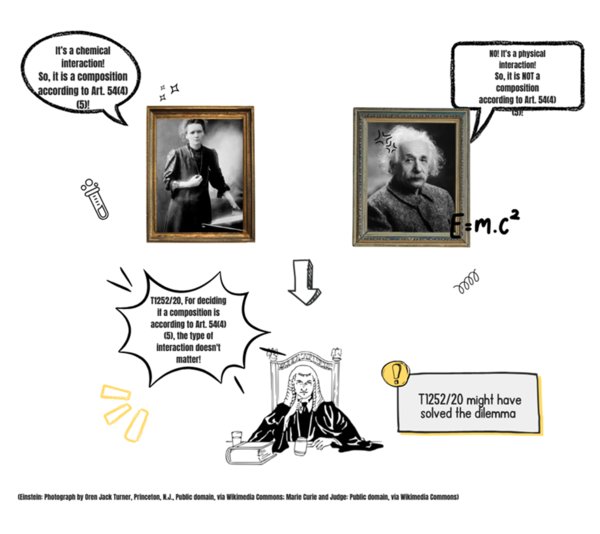
T 1252/20 provides patentee-friendly guidance on the distinction between “substances and compositions” and “devices” in the context of medical use claims. This is highly relevant, since Articles 54(4)/(5) EPC suggest that it is only “substances and compositions” which qualify for protection as medical use claims.
G 05/83 explained that a "substance or composition" in Article 54 EPC should be understood to mean an "active agent" providing a therapeutic effect in a medicament. Previous decisions have interpreted G 05/83 to mean that only if the interaction with the body is of a chemical type can it be considered a substance or composition in accordance with Article 54(4)/(5) EPC. See e.g. T 1758/15 where a gel for which the only effect was given by the 3D structure once inside the body was considered a device and thus not a “substance or composition” according to Article 54(4)/(5) EPC.
This existing practice has been overturned in T 1252/20, where the claim defined a peptide forming a hydrogel. Despite the patentee arguing that the chemical interaction between the molecules gave the 3D structure, the examining division concluded that the peptide had a physical mode of action and was thus a device, not a substance or composition. The Board reversed this decision, and at reason 9.2.3 did not consider G 05/83 to provide any legal basis for using the mode of action of a product as the relevant criterion for judging whether the product is a substance or composition:
"if the substance itself is the key element in the therapeutic, surgical, or diagnostic method, the ratio decidendi of G 05/83 is applicable to such substances, irrespective of their mode of action."
Instead, the Board found the lack of any "device-like features" of the product, e.g., shape, as demonstrating that the product was not a device (reason 7). A product may therefore be considered a "substance or composition" in a medical use claim if the claim a) defines the chemical composition of the product and/or b) does not define device-like features of the product (e.g., shape). The peptide was defined by its chemical composition, in the form of its amino acid sequence, and was a shapeless liquid without any device-like features. It was considered to be entitled to protection as a medical use claim.
Patentees will welcome this decision as it removes the complex issue of the mechanism of action from the equation, making it easier to obtain medical use claims.